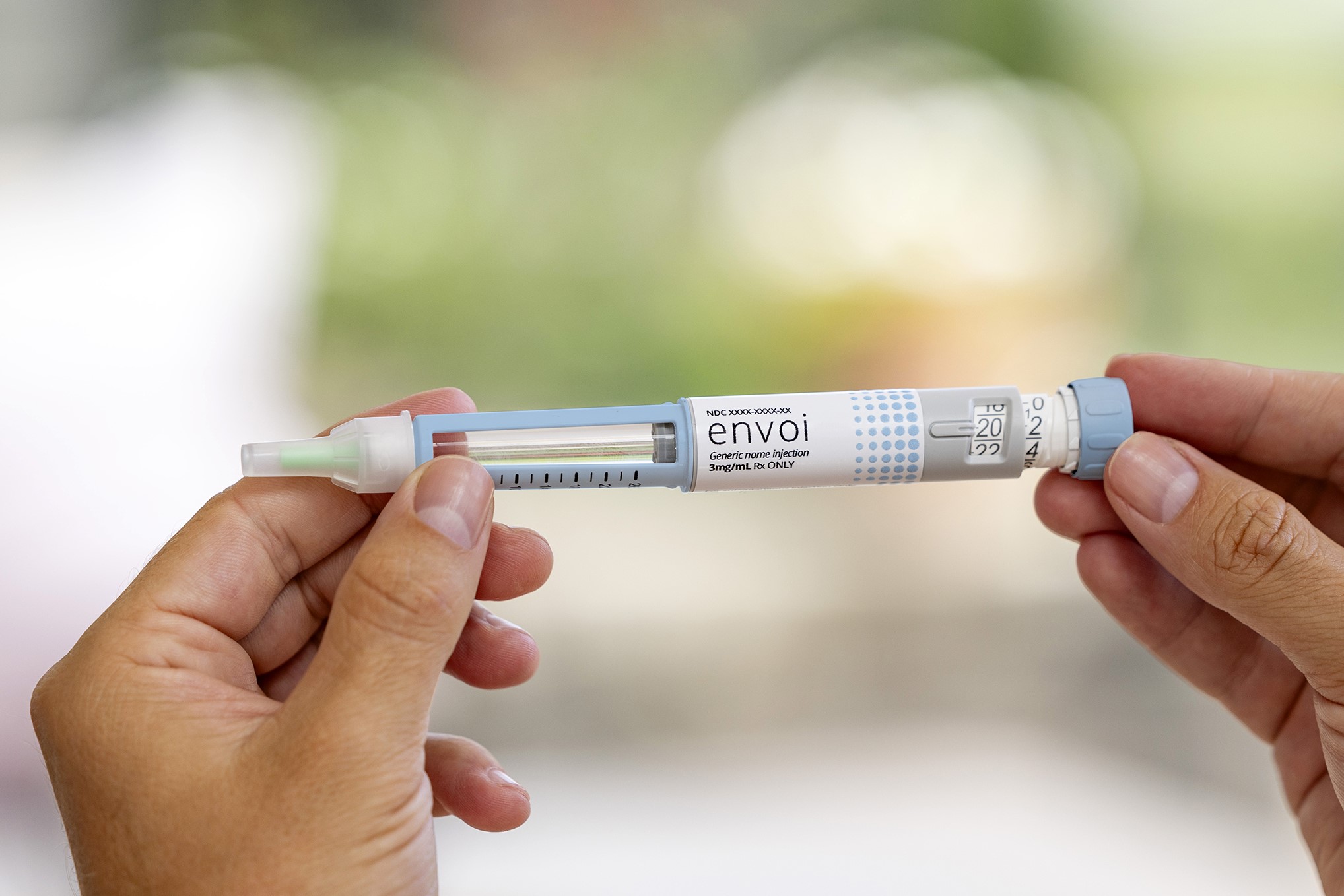
Phillips Medisize, a trusted, high-quality, high-volume company, brings confidence to patients with the Envoi Pen Injector. This familiar prefilled pen for multiple therapies with variable or fixed dosing. Envoi’s compact form, minimal elongation and low dosing force ensure that the drug delivery is easy and comfortable for all.

A disposable pen for multiple therapies
This ready-to-go solution offers all of the advantages expected from state of the art pen designs, while benefiting from our decades of device development and large-volume manufacturing expertise to reduce the challenges of bringing affordable drugs and drug-delivery devices to market.
Sample devices currently are available to support technical evaluation and human factors studies, with clinical devices scheduled to be available by the end of 2023.
| Envoi Pen Injector Features | Details |
|---|---|
| The customer can customize the pen to meet requirements for therapies including: |
|
| Customer-specified dose size: |
|
| Color: |
|
| Key assumptions: |
|
| Technical documentation: |
|
Convenient size


Excellent patient experience

Step 1
Remove cap

Step 2
Mount needle

Step 3
Dial up and dial down to select dose and prime

Step 4
Insert, inject, remove

We support our customers’ journey
For the U.S. Food & Drug Administration (FDA) and European Medicines Agency (EMA) submissions we deliver:
- Technical specifications for pen components are provided to support customers in development of pen and cartridge assembly lines
- Part build services available for stability studies
- Our customers remain the marketing authorization holder for their drug-device combinations
- Technical files are prepared that customers can reference in their drug approval applications with the FDA and EMA
Technical files can be prepared for other regions and regulatory bodies.
Get started with the Phillips Medisize pen injector platform
Learn more about the pen injector platform capabilities and our industry research.